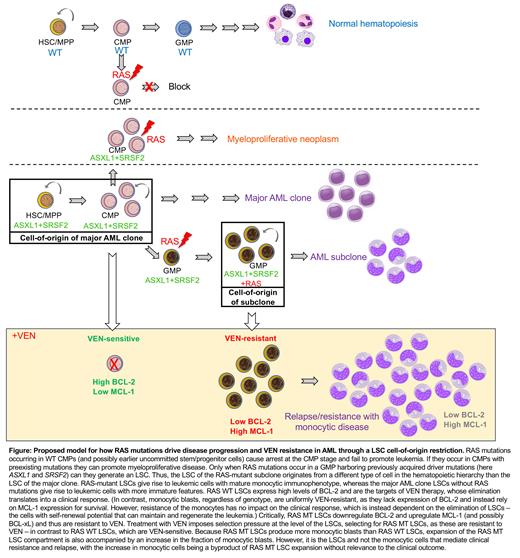
Driver mutations in acute myeloid leukemia (AML) often exhibit distinct temporal acquisition patterns, but the biological basis for this, if any, remains unknown. RAS mutations occur invariably late in AML, upon progression or relapsed/refractory disease, in contrast to their function as early drivers in solid tumors.
We developed synthetic leukemogenesis models in human induced pluripotent stem cell (iPSC)- and primary cord blood (CB)-derived human hematopoietic stem/progenitor cells (HSPCs) by introducing the prototypical NRAS G12D mutation alone or with other mutational combinations, using CRISPR, and engraftment of a transplantable leukemia into NSG mice as the readout. RAS mutations alone were not sufficient for leukemogenesis, but required specific cooperating mutations. Subsequently, by creating temporally controlled engineered models, we found that RAS mutations are obligatory late events that can only drive leukemic transformation when acquired after, but not before, cooperating mutations.
We provide the mechanistic explanation for this in a requirement for mutant RAS to specifically transform committed granulocyte-monocyte progenitors (GMPs) harboring previously acquired driver mutations, through sorting and transplantation and multi-omics experiments. In contrast, acquisition of RAS mutations in earlier progenitors (common myeloid progenitors, CMP), with or without cooperating mutations, blocked the emergence of GMPs and abolished engraftment or gave rise to a myeloproliferative neoplasm. These results indicate that advanced RAS-mutant (MT) leukemic clones have a different cell type of origin from the ancestral clone.
Next, using single-cell transcriptomics in cells isolated from xenografts of two AML-iPSC lines generated from an AML patient with a subclonal KRAS G12D mutation, one capturing the RAS WT major clone and one the KRAS G12D subclone, we show that KRAS G12D leukemia stem cells (LSCs) give rise to leukemia with a more monocytic immunophenotype, while the ancestral clone within the same patient generates more leukemic cells with primitive and neutrophil markers. These results were corroborated by Genotyping of Transcriptomes (GoT) in another AML patient with subclonal NRAS G12D mutation.
Recent studies have linked monocytic AML and, independently, RAS pathway mutations to poor responses to Venetoclax (VEN)-containing regiments. In view of the causal link we found between RAS mutations and monocytic disease, we evaluated the associations between monocytic AML, RAS mutations and response to VEN. We reanalyzed data from a cohort of 118 older/unfit newly diagnosed AML patients treated on a prospective clinical trial with VEN and decitabine (DEC) (NCT03404193). All outcomes were comparable between monocytic and non-monocytic groups, including overall response rate, duration of response (DOR) and overall survival. On the other hand, patients with N/KRAS mutations had significantly shorter DOR. These results support the presence of N/KRAS mutations and not the monocytic stage as predictors of inferior responses to VEN regimens.
To definitely test this, we treated defined cell types generated from RAS WT and RAS MT iPSC lines - CD34+ LSC-like and CD11b+/CD68+/CD14+ monocytic cells - with VEN. In agreement with previous studies, monocytes were resistant and RAS WT LSCs were sensitive to VEN. However, strikingly RAS MT LSCs were VEN-resistant. Consistent with these, single-cell transcriptomics in patient-derived AML-iPSC cells in vivo, GoT in primary AML cells and bulk transcriptomics in synthetic iPSC-AML cells showed downregulation of BCL2 and upregulation of MCL1 in the RAS MT LSCs.
This study provides the first example of a mechanistic explanation of why the timing of mutational acquisition in AML is so strongly biased. It shows for the first time that interaction between an oncogenic driver and the non-genetic developmental hematopoietic hierarchy imposes a specific LSC cell-of-origin restriction and in turn shapes the hierarchical structure of the resulting AML and critically determines therapeutic outcomes in patients. Importantly, our results have direct implications for clinical practice, as they support that RAS MT LSCs drive clinical resistance to VEN and that treatment with VEN in patients with RAS mutations can accelerate disease progression by selection of RAS MT LSCs (Figure).
Disclosures
Maiti:Lin BioScience: Research Funding; Celgene: Research Funding. Landau:Abbvie: Consultancy; AstraZeneca: Consultancy; Illumina: Consultancy, Research Funding; Mission Bio: Membership on an entity's Board of Directors or advisory committees; Pangea: Membership on an entity's Board of Directors or advisory committees; Alethiomics: Membership on an entity's Board of Directors or advisory committees; C2i Genomics: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; 10X Genomics: Research Funding; Ultima Genomics: Research Funding. DiNardo:AbbVie/Genentech: Honoraria; Astellas: Honoraria; Schrödinger: Consultancy; Notable Labs: Honoraria; ImmuniOnc: Honoraria; Fogham: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Servier: Honoraria; BMS: Honoraria. Konopleva:Abbvie, Allogene Therapeutics, Cellectis, Forty Seven, Gilead Sciences, Genentech, Sanofi, MEI Pharma, Rafael Pharmaceuticals, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini, Precision BioSciences.: Research Funding; Reata Pharmaceuticals.: Current holder of stock options in a privately-held company, Patents & Royalties; AbbVie, Forty Seven, Precision Biosciences, Gilead Sciences, Genentech, Janssen, Sanofi, MEI Pharma, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini.: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal